Coronavirus: Cordoba’s therapy protocol is described in detail
The investigator in charge of the trial with nebulizable ibuprofen assures that there are controls and it complies with regulations. The Cordoba Medical Council criticized the haste in releasing the study.
Last week, the first results of a treatment developed in Córdoba to treat patients with Covid-19 were released.
It is a nebulizable ibuprofen formulation that aided recovery in the first five patients who agreed to participate in a compassionate use protocol.
The drug was developed by researchers from the company Química Luar and the Center of Excellence in Products and Processes of Córdoba (Ceprocor), which depends on the provincial government.
The authorities and scientists were enthusiastic about the results, but pointed out that they are preliminary and involve few patients. The issue had high repercussion in the local and national media.
However, this week the Medical Council of the Province of Cordoba (CMPC) criticized the Province’s haste to show a treatment as successful without yet having gone through all the trials and regulations needed to approve a new drug.
In a letter addressed to Diego Cardozo, Minister of Health of Córdoba, Andrés de León, president of the CMPC, stated that the procedure “has not been made explicit and there are no elements to prove that the Anmat regulations for the compassionate use of medicines have been respected.
Néstor García, a clinical researcher at Conicet, is the coordinator of the patient studies. He assured that no regulations have been violated and that the mechanism for expanded compassionate use with which the treatment is being tested is in line with what the National Administration of Medicines, Food and Medical Technologies (Anmat) says.
“In medical research there are lots and lots of controls even in cases of compassionate treatment,” he explained. He specified that the procedure must be approved by the bioethics committee of each health institution and detailed that the provincial authorities request reports from the patients treated every seven days, while in a normal clinical trial they are required every three to six months.
The CMPC warned that “the indications and recommendations of the most important research and control centers in the world for the incorporation of new therapeutics have not been completed.
Garcia recognizes that the gold standard in new drug trials is the double-blind experiment compared to placebo. In this procedure, patients are divided between those who receive the drug and those who receive a placebo. This information is not known to either the patient or the physicians.
But Garcia explained: “All drugs are first tested in a small group of patients. It happened with enalapril, which is now massively indicated to treat hypertension. Remdesivir, which is being used in patients with Covid-19, was first tested in a compassionate treatment and then moved to phase one clinical trials.”
More cases
“The research is in an early period with too few patients to be able to demonstrate its effectiveness,” reads the CMPC letter. Garcia agreed with this statement.
He explained that the Province, through an external committee, has decided to test the formulation in groups of 40 patients to evaluate the results.
“To have statistically valid data to evaluate efficacy we are going to need at least about 120 patients. Based on that data, the provincial authorities could relax its use if it really serves as a treatment for Covid-19,” he says.
Special regulation
The Province created the figure of “extended compassionate use”.
Resolution. Resolution 391 of the Ministry of Health enables the Extended Compassionate Use (ECU) protocol within the framework of the pandemic emergency.
Difference. Compassionate use enables the possibility of administering a treatment not approved by Anmat to a patient, upon presentation of documentation. The UCA enables the administration of an unapproved treatment to a group of consenting patients.
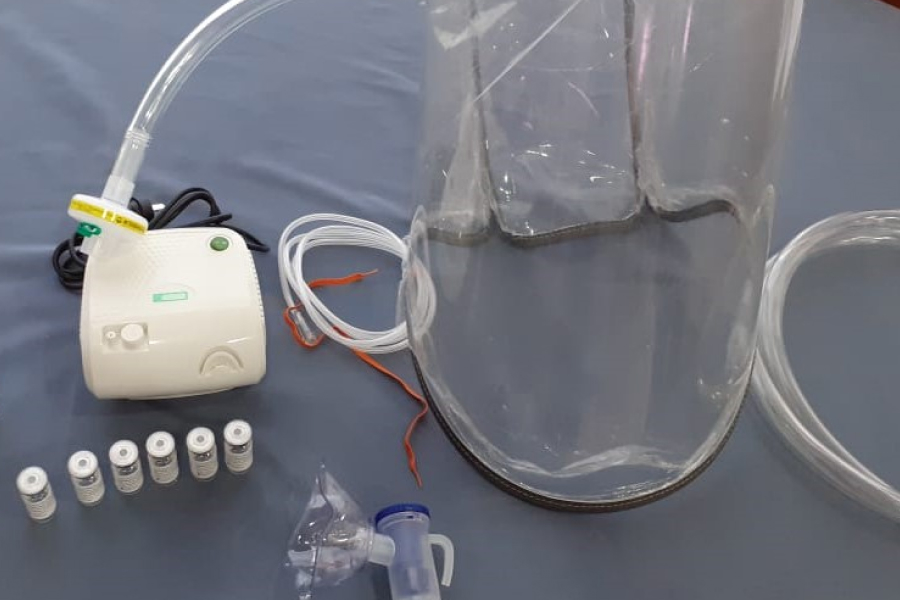
Treatment with nebulizable ibuprofen is as follows
Ibuprofen is known to be an excellent anti-inflammatory. In the case of the nebulizable formulation developed in Córdoba, this therapeutic action is key in patients with Covid-19, since complications arise precisely because of an exacerbated inflammatory process.
But in addition, in vitro studies and modeling support the idea that the drug can also inactivate the virus present in the respiratory tract and also block its entry into cells.
“The doctors who are on the front line are surprised, especially because the patients recover quickly. That makes them have confidence in the drug,” said Néstor García, Conicet researcher in charge of coordinating the trials on patients.
The treatment uses piston nebulizers that produce smaller particles to reach the lungs. Five milliliters of hypertonic ibuprofenate are placed and nebulized for 20 minutes every eight hours.
Some patients experience itching and coughing, in addition to the salty taste. Pulmonary secretions may be eliminated in the first nebulizations.
Garcia explained that patients with Covid-19 are divided into three groups: non-hospitalized cases, intermediate cases requiring hospitalization and the most severe cases. “In this trial we are not treating patients in the latter group,” he clarified.
The trial is divided into two groups. One composed of outpatients with mild symptoms and no risk factors. They are tracked by phone with an application and a questionnaire. Some of these patients had anosmia (loss of smell). The symptom was disappearing and they were negative to the Sars-Cov-2 test before.
“This is understandable because the recovery of the nerve endings linked to the sense of taste takes time. The same thing happens in other pathologies: the pathogen disappears before the symptoms do,” García explains.
The second group is composed of older adult patients with underlying diseases such as hypertension, cancer and diabetes. Before treatment, they presented changes in chest tomography and biochemical values such as D-dimer and ferritin that were reversed after treatment, Garcia explained.
An example: a 78-year-old patient with 92 percent oxygen saturation, then reached 94 percent with the assistance of an oxygen whisker. That day began with nebulizations. “After five days, the saturation reached 98 percent and they decided to withdraw assistance. One week later, the level was at 96% without mustachiotherapy. Four days later, he was discharged, after two negative tests for Sars-Cov-2,” García explained. In other words, 16 days passed between the start of treatment and discharge.