Belbarmicina INL
(tobramycin 300 mg)
First and only tobramycin for inhalation without cold chain

About Belbarmicina
It is an extemporaneous, sterile and non-pyrogenic preparation formulation of tobramycin, specifically prepared for use in infections caused by tobramycin-sensitive germs.
Tobramycin in solution is unstable, so commercially available tobramycin sulfate solutions should be stored with a cold chain. This makes it difficult to guarantee the integrity of the active ingredient.
Belbarmicina INL is the only product on the market containing tobramycin sulfate that does not require a cold chain or preservatives, since the active ingredient is presented as a lyophilized powder, which guarantees the stability of the formulation and avoids the appearance of degradation products in the formulation.

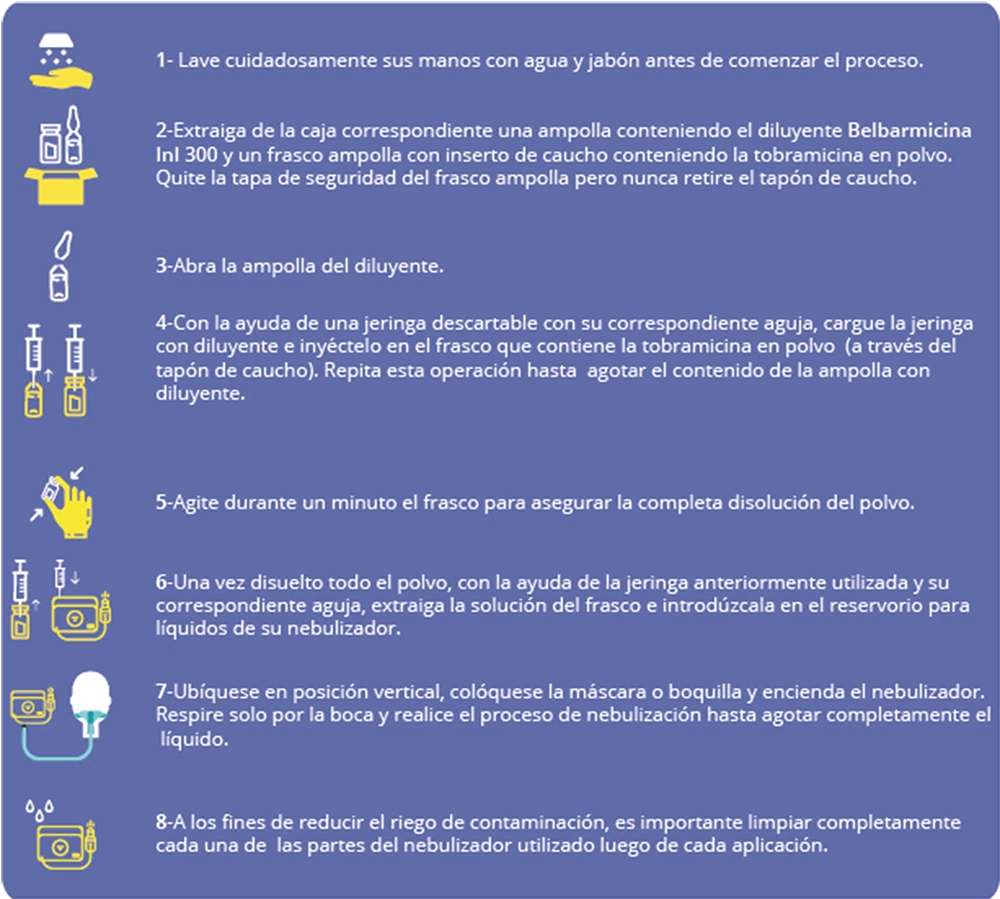
Cystic Fibrosis Treatment
Cystic Fibrosis is a genetic disease (I), generally characterized by chronic obstructive disease (COPD) and pancreatic insufficiency.
Tobramycin is a broad-spectrum antibiotic. Tobramycin sulfate is currently used in the treatment of Cystic Fibrosis caused by P. aeruginosa, H. influenzae and S.aureus, formulated as an inhalation solution.
The evaluation of the effectiveness of inhalation tobramycin, administered in two daily doses of 300 mg/5ml for 4 weeks in the treatment of Cystic Fibrosis, showed that it was adequately tolerated by the patients and improved their pulmonary function, reducing the risk of hospitalization of the patients.
Research has shown that the concentration of tobramycin is higher at pulmonary level when the drug is administered by inhalation compared to oral administration.
Advantages of extemporaneous preparation
Tobramycin is a stable molecule in the solid state; however, in the liquid state at room temperature it shows low stability. Low temperatures slow down this process. Therefore, extemporaneous preparation is ideal: tobramycin is completely solubilized in less than one minute and can be prepared at the time of use, without the risk of loss of antibiotic potency or generation of degradation products.
Belbarmicina INL, being a lyophilized powder, is a safe product that does not need a cold chain, making possible its distribution and transportation without risks or extra costs. Once reconstituted, a solution is obtained to be administered by nebulizer.
Maximum antibiotic potency
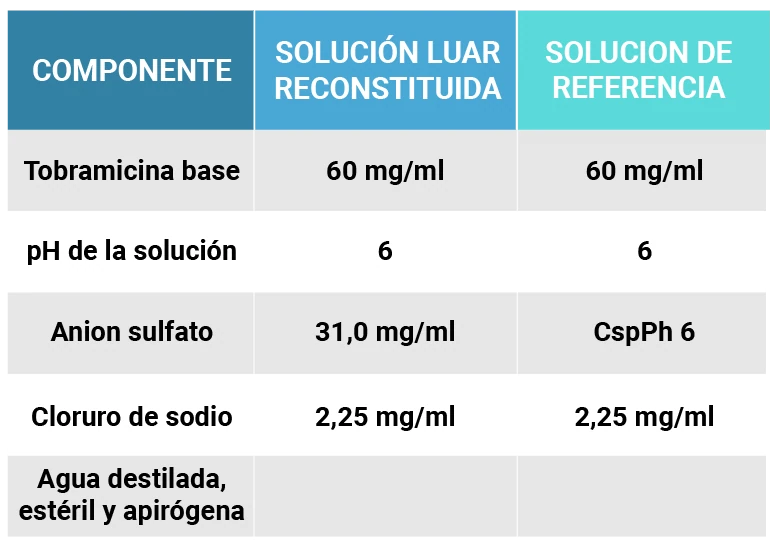
The efficacy of Belbarmicina INL is proven. The National University of Córdoba carried out the evaluation of the pharmaceutical equivalence between Belbarmicina INL and the reference product, for which physical-chemical parameters were determined that allowed the conclusion to be reached:
“The Luar solution once reconstituted has the same active ingredient, at the same concentration, the same non-active ingredients and the same dosage form as the original product. The former should be considered similar to the latter, and consequently both solutions are equivalent for pharmaceutical use.”
Research Group “Pharmacotechnics”, Dr. Rubén Manzo; Dept. Pharmacy, Fac. of Chemistry, Universidad Nacional de Córdoba.
Frequently Asked Questions
Belbarmicina INL 300 should be administered for inhalation use, in 28-day cycles, in two daily doses of 300 mg/5ml, followed by 28 days without the use of the drug.
Each extemporaneous preparation unit consists of:
– One butyl rubber insert vial containing 300 mg of trobramycin (457 mg as tobramycin sulfate).
– One glass ampoule containing 5ml of diluent (11.25 mg sodium chloride in sterile, non-pyrogenic water).
The boxes contain 28 or 56 units of vials and 28 or 56 units of diluent respectively. It is important to note that the formulation does not contain preservatives.
Store in original container in a cool, dry place, away from light, at room temperature (between 15 and 30 °C) until the expiration date of the product. No refrigeration is required. Once the solution is constituted, store at refrigerator temperature (between 2 and 8 °C), always using it within 96 hours of reconstitution, without freezing.